
The Ultimate Guide to Wastewater Neutralization for Environmental Resilience
Introduction
Neutralization is a common practice in wastewater treatment and waste stabilization. If a waste stream is found to be hazardous because of corrosivity, neutralization is the primary treatment.
Moreover, neutralization is used as a pretreatment system before a variety of biological, chemical and physical treatment processes.
Since many chemical treatment processes, such as metal precipitation, coagulation, phosphorus precipitation and water softening are pH-dependent, the pH of these processes is adjusted to achieve maximum process efficiency.
Furthermore, the pH of the effluent wastewater from different industrial activities also requires adjustment before its discharge into receiving water bodies.
The US EPA has set pH standards for different types of water; for example, the pH range required to protect marine aquatic life is 5–9.
Neutralization is the process of adjusting the pH of water through the addition of an acid or a base, depending on the target pH and process requirements.
Some processes such as boiler operations and drinking water standards need neutral water at a pH of 7.
General Considerations for neutralized wastewater .

One significant requirement is the adoption of more stringent regulatory requirements for treated wastewater discharge. These requirements aim to protect the environment and public health by setting limits on the concentration of pollutants and contaminants in discharged water.
To meet these requirements, treatment facilities must ensure that their neutralization processes are effective and efficient, and that the quality of treated water meets regulatory standards.
Another important update is the integration of advanced treatment technologies to further improve the quality of treated water. This includes the use of membrane filtration, reverse osmosis, or UV disinfection, which can effectively remove remaining pollutants and contaminants from neutralized wastewater.
These technologies are particularly useful in treating wastewater from industries with high pollutant loads, such as chemical or pharmaceutical manufacturing.
Furthermore, there has been a growing emphasis on promoting environmental sustainability in neutralized wastewater treatment. This includes exploring ways to minimize energy consumption, reduce greenhouse gas emissions, and recover valuable resources from wastewater.
For example, anaerobic digestion can be used to generate biogas from organic matter in wastewater, which can then be used as a renewable energy source.
In addition to these updates, there is a focus on promoting public awareness and engagement in the neutralization of wastewater. This includes educating the public on the importance of proper disposal of household chemicals and pharmaceuticals to prevent contamination of wastewater, as well as encouraging community involvement in water conservation efforts.
Overall, the latest updates in general considerations for neutralized wastewater treatment aim to improve compliance with regulatory requirements, enhance the quality of treated water, promote environmental sustainability, and increase public awareness and engagement in water conservation efforts.
Parameters To be Evaluated

In neutralization, several parameters need to be assessed and evaluated before the actual pH adjustment is carried out.
These parameters are discussed in the following sections.
Acidity and Alkalinity

Evaluating acidity and alkalinity parameters for neutralizing wastewater have focused on the development of more accurate and efficient measurement techniques.
These advancements aim to provide a comprehensive understanding of the acid-base balance in wastewater, enabling better control and optimization of the neutralization process.
One notable development is the use of automated titration systems that can precisely measure and analyze the acidity and alkalinity levels in real-time.
These systems employ advanced sensors and algorithms to monitor the pH, as well as the concentration of acids and bases, allowing for more precise dosing of neutralizing agents.
Furthermore, there has been a shift towards utilizing online monitoring systems that continuously measure acidity and alkalinity parameters throughout the treatment process.
This real-time data enables operators to make prompt adjustments and ensure optimal neutralization, preventing under or over-treatment of wastewater.
In addition to technological advancements, there is a growing emphasis on adopting sustainable approaches in neutralizing wastewater.
This includes exploring alternative neutralizing agents that are eco-friendly and cost-effective, such as certain organic compounds or waste byproducts from other industries.
Overall, these recent updates in evaluating acidity and alkalinity parameters contribute to enhanced efficiency, accuracy, and sustainability in the neutralization of wastewater.
By leveraging advanced measurement techniques and embracing eco-friendly solutions, we can improve wastewater treatment processes while minimizing environmental impact.
Buffer Capacity
The word “buffer” stands for stubbornness against any change. Buffers are always defined in the context of pH in environmental chemistry.
pH buffers are those that resist any changes in solution pH when an acid or a base is added to the solution.
They play a critical role in chemical neutralizing procedures.
Buffers generally contain a mixture of a weak acid and its salts (conjugate base) or weak bases and their conjugate acid.
A solution buffered at a particular pH will contain an acid that can react with an externally added base and vice versa.
The overall efficiency and chemical cost of the neutralization process depend on the presence of pH buffers in wastewater.
In natural waters and wastewaters, the buffering capacity arises due to the presence of phosphates, carbonates and other weak organic acids.
The mineral composition of natural waters is regulated by a buffer system involving natural clay minerals such as Illite and kaolinite.
Careful consideration should be given while neutralizing such waters.
If the buffering capacity of the water or wastewater to be neutralized is not considered, the actual amount of neutralizing chemical required may vary widely and causes operational problems.
Hardness
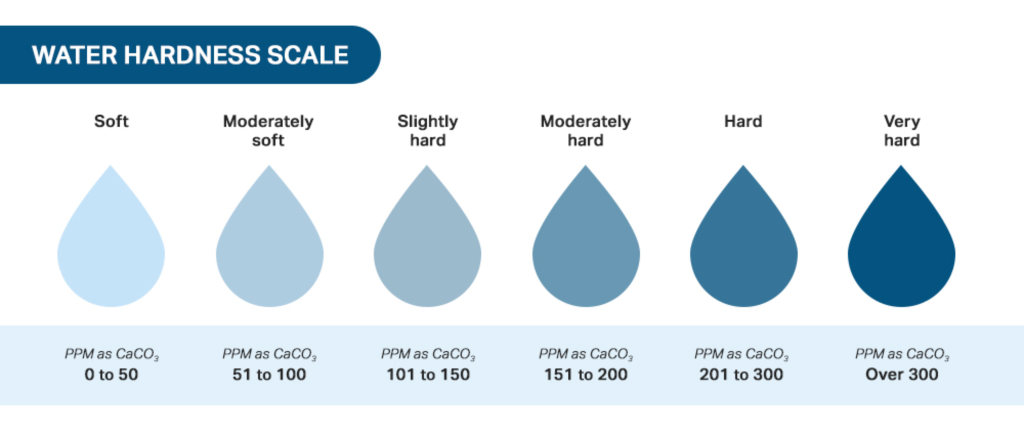
The last updates regarding hardness in wastewater indicate that it continues to be a significant concern in the process of neutralization.
Hardness refers to the concentration of calcium and magnesium ions in water, which can cause scaling and other issues in industrial processes.
To address this, various techniques such as chemical precipitation ( Read more about chemical precipitation ) , ion exchange, and membrane filtration are being employed to effectively reduce hardness levels in wastewater.
These advancements aim to improve the efficiency and effectiveness of neutralization processes while minimizing the negative impacts of hardness on industrial operations and the environment.( Read more about Hardness Removal )
PH

Evaluating pH parameters for neutralizing wastewater involve the use of advanced sensors and automated monitoring systems.
These technologies allow for more accurate and efficient pH measurements, which are crucial in determining the appropriate amount of neutralizing agents needed to treat wastewater.
Additionally, there has been a growing trend towards using more sustainable and eco-friendly solutions for wastewater treatment.
This includes the use of natural materials such as limestone and oyster shells as neutralizing agents, which can help reduce the environmental impact of the treatment process.
Overall, these advancements in pH evaluation and sustainable treatment options are helping to improve the efficiency and effectiveness of wastewater treatment, while also promoting environmental sustainability. ( Read more about PH adjustment for better performance )
Neutralization practices

Neutralization practices for wastewater treatment have interested by improving efficiency, sustainability, and cost-effectiveness. These updates aim to enhance the overall effectiveness of neutralization processes while minimizing environmental impact.
One significant update is the adoption of advanced dosing control systems. These systems utilize real-time monitoring and feedback mechanisms to accurately measure and regulate the dosage of neutralizing agents.
By optimizing the dosing process, operators can achieve precise pH adjustment and minimize the use of chemicals, reducing operational costs and minimizing the generation of sludge or byproducts.
Another important update is the integration of automated pH monitoring and control systems. These systems continuously monitor the pH levels of the wastewater and automatically adjust the dosage of neutralizing agents as needed.
This allows for more efficient and consistent neutralization, reducing the risk of under or over-treatment.
Furthermore, there has been a shift towards utilizing sustainable and eco-friendly neutralizing agents. Natural materials such as limestone, oyster shells, or certain organic compounds are being explored as alternatives to traditional chemicals.
These materials not only effectively neutralize wastewater but also contribute to environmental sustainability by minimizing chemical usage and reducing waste generation.
In addition, there is an increasing focus on the integration of renewable energy sources into neutralization processes.
This includes utilizing solar power or bioenergy for powering treatment facilities, reducing reliance on fossil fuels and lowering carbon emissions.
Overall, the latest updates in neutralization practices for wastewater treatment emphasize improved efficiency, sustainability, and cost-effectiveness.
By adopting advanced dosing control systems, automated monitoring and control, sustainable neutralizing agents, and renewable energy sources, we can enhance the effectiveness of neutralization processes while minimizing environmental impact.
References
[1] W. Stumm and J. J. Morgan, Aquatic Chemistry, John Wiley and Sons, New York, 1981.
[2] J. P. Chen and L. Wang, Characterization of a Ca-alginate based ion exchange resin and its applications in lead, copper and zinc removal. Separation Science and Technology, 36(16),3617–3637 (2001).
[3] F. N. Kemmer, The Nalco Water Handbook, McGraw-Hill, New York, 1988.
[4] L. K. Wang, Y. T. Hung, and N. S. Shammas (eds.), Physicochemical Treatment Processes. Humana Press, Totowa, NJ (2005).
[5] M. L. Davis and D. A. Cornwell, Introduction to Environmental Engineering, 3rd ed., McGraw-Hill, New York, 1998.
[6] C. A. Hazen and J. I. Myers, Neutralization tactics for acidic industrial wastewater. In: Process engineering for Pollution Control and Waste Minimization (D. L. Wise, ed.), Marcel Dekker, New York, 1994.
[7] US EPA, An Appraisal of Neutralization Processes to Treat Coal Mine Drainage. EPA- 670/2-73-093, U.S. Environmental Protection Agency, Washington, DC, 1973.
[8] US EPA, Design Manual—Neutralization of Acid Mine Drainage, U.S. Environmental Protection Agency, Municipal Environmental Research Laboratory, EPA-600/2-83-001, U.S. Environmental Protection Agency Technology, Cincinnati, OH, 1983.
[9] US EPA, Evaluation of Flow Equalization at a Small Wastewater Treatment Plant, US Environmental Protection Agency, Municipal Environmental Research Laboratory, EPA- 600/2-76-181, U.S. Environmental Protection Agency, Cincinnati, OH, 1976.

