
Positives & Negatives of Ion Exchange in Removing Groundwater Contaminants
By removing undesirable dissolved ions from groundwater, both cations (positively charged) and anions (negatively charged), ion exchange is a chemical purification method that uses specially made resins to replace them with less hazardous ions and enhance water quality. Negatively charged water in a typical system is defined as water that contains anionic contaminants like nitrate, sulfate, or chloride, which, if left unchecked, can degrade the drinking water’s safety and aesthetic appeal. In a regulated exchange process, ion exchange resins that are specific to particular ions work to draw these impurities and release innocuous ions like sodium or hydroxide.
Efficient Elimination of Specific Groundwater Pollutants
A wide range of dissolved pollutants can be effectively eliminated from groundwater supplies using ion exchange. Its ability to get rid of hardness-causing cations (like Ca2+ and Mg2+) as well as more problematic species like arsenic (As(V)), nitrate (NO₃⁻), perchlorate (ClO₄⁻), radium, uranium, and hexavalent chromium are some of its advantages. Positively charged metal ions are trapped by cation-exchange resins, whereas negatively charged water contaminants, such as nitrate or sulfate, are captured by anion-exchange resins. Because of this, the process is very adaptable, with resin types chosen according to the contaminant profile of a site.
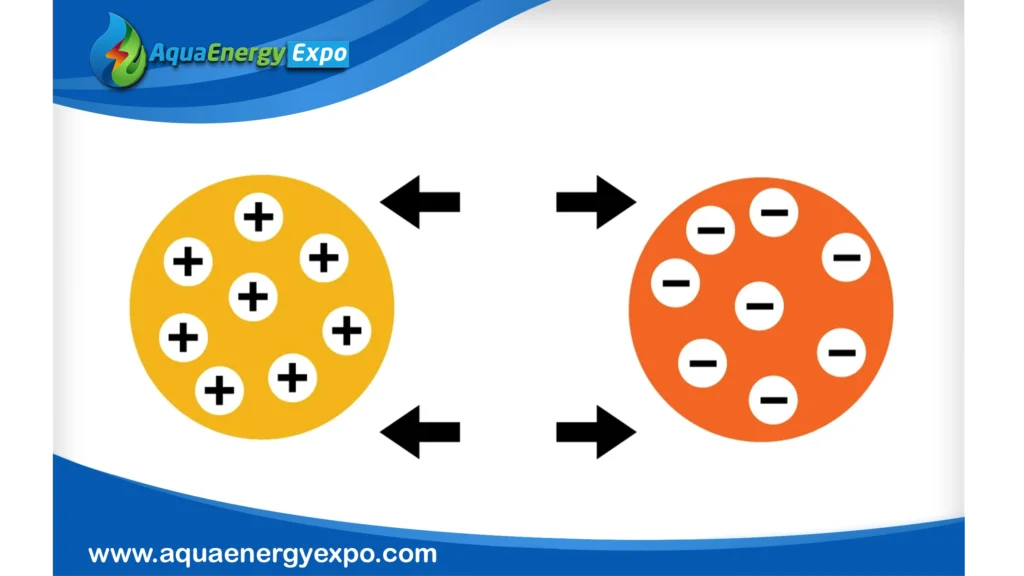
Ion Exchange Resin Types
Ion exchange resins can be made industrially or synthetically. Resins are microporous beads composed of polystyrene and polyacrylate. They are made up of tiny, microporous beads that are between 0.3 and 1.3 millimeters in size. Ions in the resin interact with ions in the water when water passes through the beads. This is how the water absorbs pollutants.

Industrial water treatment resins fall into four main categories:
Strong Acid Cation (SAC) Resins:
SAC resins change salts into their corresponding acids by neutralizing strong bases. They are able to eliminate all cations and substitute hydrogen ions for them. All pH ranges are covered by SAC resins.
Strong Base Anion (SBA) Resins:
SBA resins change salts into their corresponding bases and neutralize strong acids. These are frequently used in demineralization and water softening procedures.
Weak Base Anion (WBA) and Weak Acid Cation (WAC) Resins:
These resins both neutralize strong acids and bases. They are employed in dealkalization systems and partial and total demineralization procedures. Carbonic acid is produced by WAC resins by removing cations, but most water supplies contain more cations than they can eliminate. They are more popular than SAC resins because of their high regeneration efficiency, which reduces waste. Acids like hydrochloric, nitric, and sulfuric acids are eliminated by WBA resins.

Choosing the Right Ion Exchange Resin
The resin manufacturer should be consulted in order to choose the best ion exchange resin for the task. Check for hardness, sodium, sulfate, chloride, alkalinity, pH, conductivity, and other problematic ions in the water that needs to be treated. The resin manufacturer can recommend a process and choose a resin if you provide these test results along with the desired treated water quality.
| Contaminant |
Ion Type & Occurrence |
Removal by Ion Exchange |
Key Challenges / Notes |
| Boron | Present as borate (weakly ionized anion); from agricultural runoff | Poorly removed by standard anion resins or reverse osmosis | Requires boron-selective resin (e.g., methyl-glucamine functional) which is expensive but effective |
| Nitrate & Nitrite | Common anions in agricultural runoff | Removed by nitrate-selective anion resins (triethyl-/tributyl-amine); non-selective resins risk “dumping” | Nitrite removal incomplete; requires oxidation to nitrate pre-treatment; risk of “nitrate dumping” if resin is over-run |
| Transition & Heavy Metals | Positively charged cations | Effectively removed with standard, weak acid, or chelating cation resins | Resins work in narrow pH, impacted by competing ions; metals may form complexes making ion removal challenging |
| Radioactive Metals | Uranium (anion, pH-dependent), radium (cation) | Well removed by conventional resins; oxidation state matters | High selectivity makes regeneration difficult—often single-use |
| Phosphate | Orthophosphate present in treated municipal water | Loads on anion resins; accumulates under alkaline regeneration | Fouling issue; requires acid regeneration |
| Aluminum | Trivalent cation (from alum usage) | Softening and deionization resins remove it | Difficult to regenerate; leads to resin fouling |
| Ammonia | Ammonium cation (NH₄⁺) in waters | Removed by softeners; initial resin cycle removes ammonia, but hardness displacement later releases NH₄⁺ | Requires multi-stage softener system (primary + polishing) to avoid NH₄⁺ breakthrough |
| Sulfate | Divalent anion with high resin affinity | Effectively removed by strong-base anion resins (Type 1 & 2 SBA) | High sulfate can displace other ions and cause “dumping” of nitrates upon exhaustion; sulfate competes heavily |
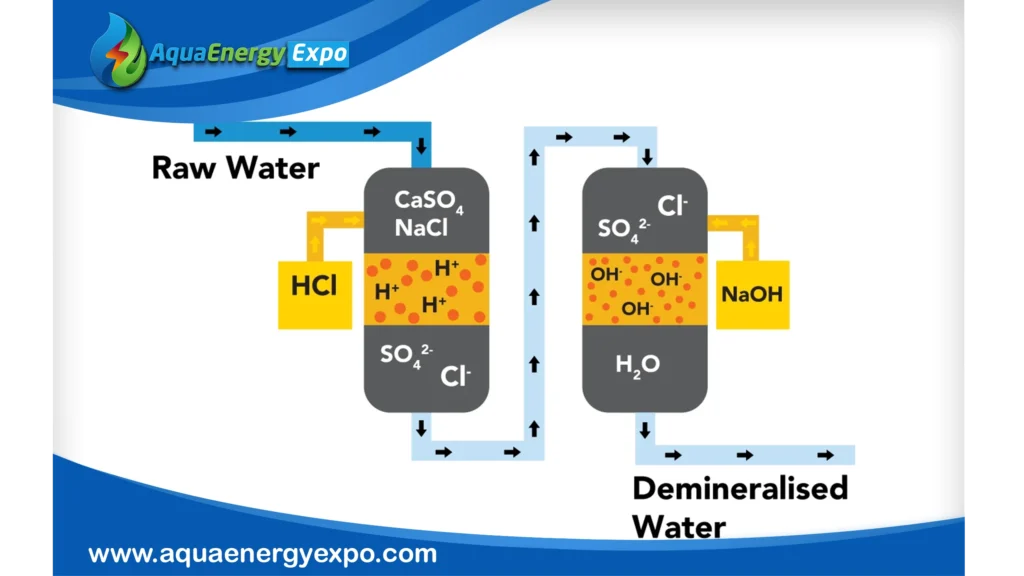
Pros: Advantages of Ion Exchange
1- Extremely Specific Elimination
Ion exchange is notable for its accuracy in eliminating specific ions, like nitrates or hardness minerals, without appreciably altering other aspects of water chemistry. This maintains healthy neutral ions and guarantees that the treatment is targeted.
2- Production of High Purity Water
High-purity systems can approach 18 MΩ, while advanced applications such as demineralization or mixed-bed systems can reduce total dissolved solids to very low levels. Resistivity can reach 50 kΩ to 1 MΩ.
3- Economical Function
Particularly when ion-specific cleanup is the goal, ion exchange systems frequently require less capital investment than alternatives like reverse osmosis. Resin bed regeneration reduces operating costs by enabling long-term reuse.
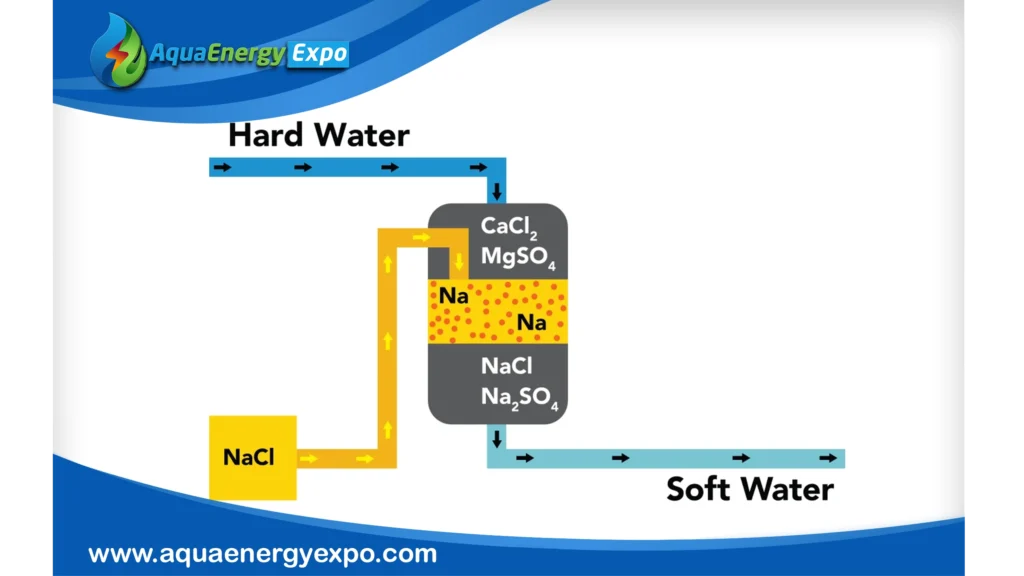
Cons: Limitations and Challenges
1- Restricted Contaminant Removal Scope
Non-ionic pollutants such as viruses, particulates, VOCs, and bacterial pathogens are not addressed by ion exchange. This indicates that extra filtration or disinfection steps are frequently required.
2- The Need for Regular Regeneration
Resins must regenerate after becoming saturated with the target ions (e.g., caustic for anion resins, salt brine for cation resins). This necessitates handling chemicals, downtime, and the production of brine or waste effluent that must be disposed of carefully.
3- The Effects of Wastewater on the Environment
Wastewater that has had its ions removed is produced by the regeneration process. The environment may suffer from improper disposal, particularly if brine discharges more than permitted.
4- Resin Fouling and Upkeep
Particulates or chemical precipitation (such as calcium on anion resins) can cause resin beds to foul, reducing performance and raising maintenance requirements.
Ion Exchange vs. Other Water Treatment Methods
Ion exchange has clear benefits and drawbacks when contrasted with other water treatment techniques like distillation, reverse osmosis, and activated carbon filtration:
| Water Treatment Method |
Advantages |
Limitations |
| Ion Exchange | Effective for water softening, heavy metals removal, customizable | Requires regeneration, not effective for organic or biological contaminants, waste disposal issues |
| Distillation | Removes a wide range of contaminants, including bacteria and viruses | Expensive, slow, not suitable for large-scale use |
| Reverse Osmosis | Effective for a broad range of contaminants, including salts and metals | Requires high water pressure, produces wastewater |
| Activated Carbon | Excellent for removing organic compounds, odors, and chlorine | Less effective for hard water and heavy metals |
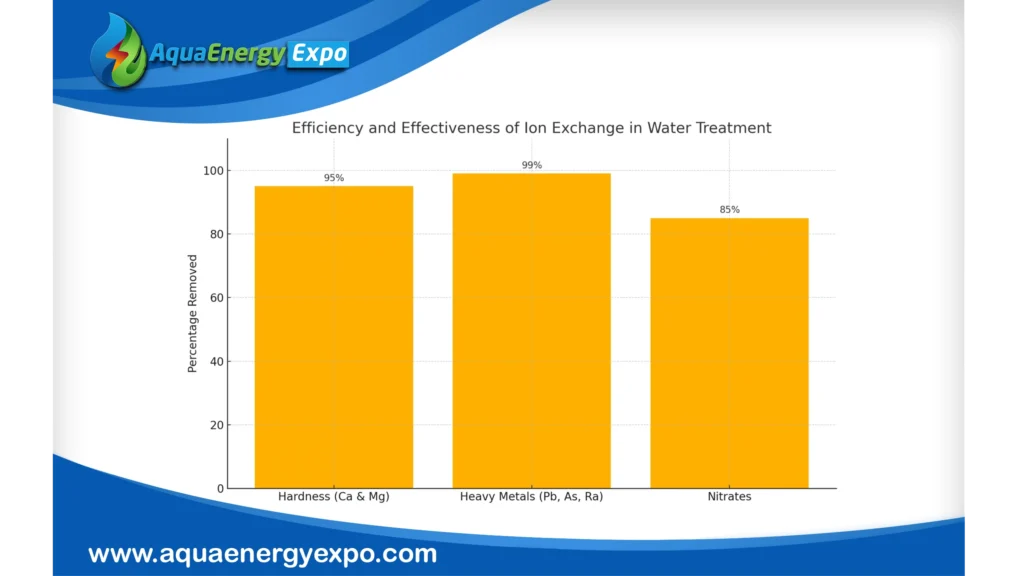
Efficiency and Effectiveness of Ion Exchange
Percentage of Hardness Removed: Ion exchange can eliminate 90–95% of the calcium and magnesium ions that cause hard water.
Elimination of Contaminants: Heavy metals like lead, arsenic, and radium can be reduced in water by up to 99% through ion exchange.
Nitrate Reduction: Up to 85% of nitrates can be effectively eliminated from tainted water sources using ion exchange systems.
Conclusion:
In summary, ion exchange resin is a powerful water filtration technique that works especially well for eliminating the minerals that contribute to hard water. Before choosing, it’s crucial to weigh the benefits and drawbacks of ion exchange resin in relation to alternative water filtration techniques. To find out which approach is best for your house and water quality requirements, speak with a water filtration specialist.
To explore the latest innovations in water and energy technologies, and discover a wide range of products and solutions from around the world, you can visit the virtual exhibition AQUA ENERGY EXPO which featuring leading companies in water treatment, desalination, and sustainable energy through the following link:
https://aquaenergyexpo.com/
References:
https://www.resin-products.com/ion-exchange-resin-pros-and-cons

